1093
Views & Citations93
Likes & Shares
The brain interstitial fluid (ISF) in the brain
interstitial space (ISS) forms external medium for the neural cells and is
involved in various vitally important processes including volume transmission,
signal transduction, coordinated response to changes in the external and
internal environments of the brain, transport of nutrient and gases, removal of
metabolic waste products. It participates in the migration malignant and stem
cells, targeted delivery of drugs etc. The ISS presents a nanodimensional structure.
This feature of the ISS has been commonly misinterpreted as an indication that
it presents a Fickian diffusional barrier to mass-transfer processes there. A
new interpretation, based on an interdisciplinary approach, states that the
brain interstitial space should be considered the brain nanofluidic domain
where fluid flow is governed by the principles of nanofluidics. The nanofluidic
approach to the brain water metabolism solves a number of problems inherent to
the diffusion barrier theory and opens new perspectives in brain physiology,
pathology and in nanomedicine.
Keywords: Brain,
Water metabolism, Interstitial system, Diffusion-barrier theory, Nanofluidics,
Nanofluidic domain, Nanofluidic mechanism
Abbreviations: ISS: Interstitial Space; ISF: Interstitial Fluid; CSF:
Cerebrospinal Fluid; NFD: Nanofluidic Domain; BBB: Blood-Brain Barrier; BCSFB: Blood-CSF Boundary;
AQP1: Aquaporin-1; AQP4: Aquaporin-4
INTRODUCTION
The neurons attract the most attention in
neurobiology; however, current knowledge of neural circuit scan only partially
explains the neurological and pathophysiological conditions of the brain. It is
also important to consider the role of brain ISS containing the ISF that bathes
the nerve cells and the neurophil [1]. It should be observed that after many
decades of research, it came to head that the interstitial space presents a
rather neglected area [2]. The ISF forms external medium for the neural cells and
is involved in non-synaptic intercellular communication (volume transmission),
signal transduction, information processing and integration, coordinated
response to changes in the external and internal environments of the brain. It
ensures nutrient and gas transport, targeted delivery of drugs and metabolites,
formation and resolution of the brain β-amyloid deposits and other metabolic
waste products. The ISF is involved in maintaining ionic homeostasis,
participates in the migration of cells (malignant cells, stem cells), transfer
of heat generated by neuractivity [1,3,4]. Dynamic and complex ISS connects the
vascular system and neural networks and plays crucial roles in brain
physiology. Investigation of the ISS can provide new perspectives for understanding
brain function and exploring new strategies to treat brain disorders. In our
era of interdisciplinary research new groundbreaking ideas may emerge from
apparently far removed non-biological disciplines. The issue of fluid movement
and mass-transfer events in the ISS seems to come to a stall in view of its
nanodimensionality [5]. However, from an interdisciplinary approach, it is the
nanodimensionality that might servea clue to solving its puzzle: the ISS
characteristic width of 20-60 nm [6] puts this water system into the realm of
nanofluidics. Nanofluidics, a rapidly developing over last two decades branch
of science, deals with the phenomena and fluid behavior in compartments of
various geometry where at least one characteristic geometrical dimension is the
range of 1-100 nm [7]. Due to domination of the surface effects, water in
BRAIN
FLUIDS AND COMPARTMENTS
The extracellular fluids of the
human brain are contained in compartments varying in size from nano- to
macro-dimentional ones. Containing nanoconfined water, the ISS occupies up to
20% of the total brain volume and falls into the category of nanodimentional
structures [2,5,6,9]. By definition, the ISS is a NFD.
The CSF, of about 11% of the
brain volume, fills the ventricular and the subarachnoid macro-compartments and
contains bulk water [10]. The parenchymal blood microvessels, occupying
1.5%-5.5% of the brain volume, present another bulk-water compartment [11]. The
nanoconfined ISF bridges the bulk water moieties of the blood and the SCF. The
exchange of water between blood and the ISF is controlled by the BBB [12]. The
CSF is in a constant to-and-fro motion driven by the oscillations of the brain
intracranial pressure [13]. The integral CSF flow might proceed in either
inward or outward direction. The CSF and the ISF present one functional moiety
of freely communicating fluid. The BBB controls water transition between the
blood bulk water and the nanoconfined water of the ISS. The BCSFB regulates
water flow between two bulk water volumes: the blood and the CSF. The
transition from the nanoconfined water of the ISS to the CSF bulk water also
takes place with water moving on over larger the extended-nano compartments
(the characteristic width from 100 nm to 1 µm), micro-compartments and
macro-compartments [14,15].
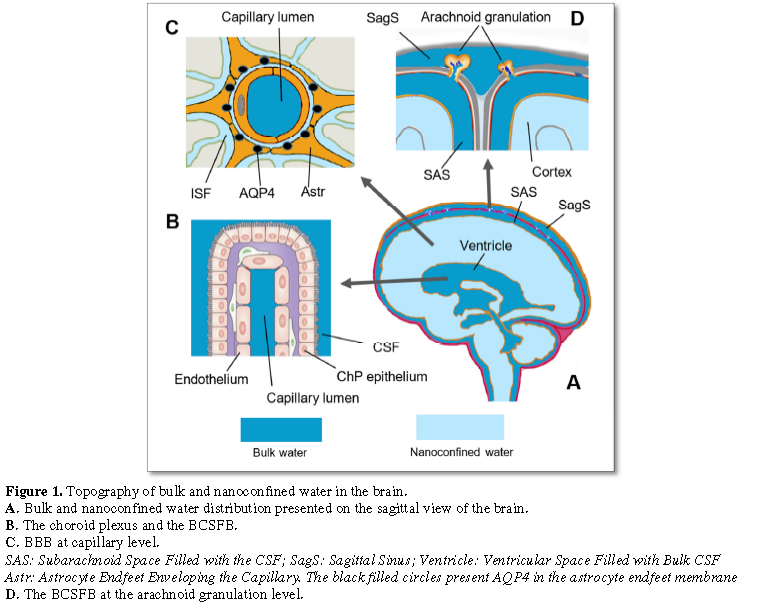
Figure
1
demonstrates the distribution of bulk and nanoconfined water in the brain.
There are at least two distribution patterns
as far as the proximity of the bulk and the nanoconfined water moieties is
concerned. One of those is the bulk/bulk water pattern observed at the BCSFB of
the choroidal plexus (Figure 1B) and
at the BCSFB of the arachnoid granulations (Figure
1D). Another pattern is presented by the bulk/nanoconfined water divide of
the BBB (Figure 1C). Figure 1A shows that two basins of bulk
water (e.g. the subarachnoid CSF and the ventricular CSF) might be
short-circuited with the nanoconfined water of the brain nanofluidic domain.
BULK WATER FLOW
ROUTES
According to the classical views, the choroid
plexus inside the brain ventricles is the main source of CSF formation. The
secreted CSF flows as a bulk fluid along the cerebral macrospaces to get
absorbed mostly into the venous sinuses through arachnoid granulations [16-18].
Apart from that CSF is absorbed into lymph flowing along the perineural spaces
to reach the lymph nodes [19,20] and via the glymphatic pathway [21-24].
A hypothesis opposing the orthodox theory
states that exchange of water occurs
everywhere in the brain parenchyma between brain capillaries, the ISF and CSF
Water is constantly formed and reabsorbed at the microvascular level and does
not flow in a unidirectional way along CSF spaces [25-27]. Contrary to the
predictions of classical theory, CSF circulation is pulsatile with the to and
fro movement throughout the entire brain. Key controlling elements in brain
water and CSF homeostasis are astrocytes and aquaporins [20].
A stumbling block of the theories of brain
water metabolism is the mechanism of fluid passage through the nanodimensional
ISS. A dominating opinion in the medical community is that the ISS, an
irregular, tortuous and narrow space among neural cells, capillaries and neurophil,
is too narrow to permit any bulk flow [9,28]. Fickian diffusion has been
considered a dominant governing mechanism there with the ISS presenting a
diffusion barrier to fluid movement [5,28,29]. Mass transfer events in the ISS
are described in terms of diffusion coefficients, gradients and ISS tortuosity
[5,30,31]. ISF drainage through the ISS is deemed to be a diffusion-driven
process [5,29].
The diffusion-barrier theory conflicts with
the experimental evidence demonstrating convection and bulk flow in the
confined fluid compartments of the brain [32-37]. There is observed very fast
water movement from artery to brain parenchyma and ventricular CSF [38]. The
small and large molecules may move with the same rate in the ISS while,
according to the diffusion theory, they should have individual effective
diffusion coefficients [23,39-41]. The orthodox views on the ISS find their
reflection in simulations of mass transfer events taking place there. These
models are built on either Darcy’s laws for fluid flow through porous media
[42-44] or Fick’ laws of diffusion [29,45,46].
Animal experiments with the use of two-photon
imaging of small fluorescent tracers demonstrate that CSF enters the parenchyma
along paravascular spaces surrounding penetrating arteries and were cleared
along paravenous drainage pathways. The bulk fluid flow between these
anatomical influx and efflux routes is controlled by water channel AQP4
expressed in the astroglia end feet at the border dividing the periarterial
compartment and the ISS [22,33,47]. This route of CSF exchange presents the
glymphatic mechanism based on fluid convection and bulk flow [48]. According to
glymphatic mechanism, the CSF bulk flow is driven by the cerebral arterial
pulsations [36]. Much prominence is given to its role in removal β-amyloid that
is believed to be involved in pathogenesis of Alzheimer disease [49-51].
The fluid flow route after glymphatic
mechanism, as well as other convectional mechanisms, includes a stage when
water enters into and passes through the ISS. At this step convection clashes
with the diffusion-barrier theory. The adherents of convection chose to
sidestep this theoretical nuisance and not to go any deeper into the
controversy. It is not that this fact did not receive due attention from other
researchers. Thus, the Verkman’s group, on modeling the glymphatic mechanism
found that unrealistically high hydrostatic pressure gradient is needed to
energize local parenchymal convective flow and fluid passage through the ISS
[43]. The results of this research might be extended to include other cases of
convection in the ISS. Incidentally, the Verkman’s group used the no-slip
Navier-Stokes equation to model water passage through the nanodimensional ISS.
The significance of this misconception is discussed further in the text.
At present, the experimental results on water
movement in the ISS facts speak against diffusion as the only mechanism of
fluid movement and mass transfer in the brain. At the same time, the nanodimensionality
of the ISS is used as an argument for the diffusion-barrier theory. The
controversy stays unresolved still pending its solution.
NANOFLUIDIC APPROACH
TO THE BRAIN INTERSTITIAL SPACE
A striking feature of the nanoconfined water
is significant enhancement of its flow rate due to the hydrodynamic surface
slip [52,53]. From the conventional point of view, it seems counterintuitive
and even unsupported as has been demonstrated in simulations based on Darcy’s
or the no-slip Hagen-Poiseuille's equations [54,55]. An interesting example of
such unexpected behavior present aquaporins, the water-conducting nanopores.
They exhibit water permeability typically three orders of magnitude higher than
follows from the classical no-slip framework for the same pore size [56]. On
the whole the flow capacity of confined water might be up to ∼107 times of that calculated with the no-slip
Hagen-Poiseuille’s equation for nanopores with various contact angles and
dimensions [57,58]. Much valuable information on water rheology in
nanoconfinement, relevant to biological systems, has been obtained using carbon
nanotubes and nanotubes manufactured from other materials [59-64]. They present
non-biological systems of nanoconfined water making it possible to get a deeper
insight into water rheology with biological implications. Water flow rates through
carbon nanotubes were comparable to the flow rates for AQP1 and were
practically independent of the length of the nanotube, in contrast to
predictions of macroscopic hydrodynamics [65]. Initially aquaporins held the
first place as far as the high water-transfer rate was concerned being an
object of professional envy and a target to achieve for nanoengineers. Finally,
this record was beaten with the use of the thin-walled carbon nanotubes [61].
We introduced the nanofluidic slip-flow approach to fluid movement in the ISS
as early as 2018 to model brain water metabolism [66]. Theoretical and
experiment-based assumptions of the model were as follows: (a) the brain
nanodimensional interstitial space presents NFD with the fluid movement there
governed by the slip-flow mechanism [18,25]; (b) aquaporin AQP4 ensures kinetic
control over water movement between the blood and the ISS [26,29,34,39]; (c)
the pulsatory intracranial pressure presents a driving force behind the
isosmotic fluid exchange between the capillaries and the interstitial space
[26,35,36,38,40]. Introducing the nanofluidic approach makes redundant the
diffusion-barrier theory with its intrinsic problems. The model demonstrated
good predictability in respect to physiology of brain water metabolism and
relevance in explaining some clinical conditions [66]. The nanofluidic approach
was used to model convective mass-transfer events in the ISS. Computer
simulation of convective transfer of glucose, oxygen and carbon dioxide, taking
place within the NFD of the brain neurovascular unit, demonstrated that this
mechanism is physiologically realistic [67]. Other volume transmission events
in the brain ISS might also find their solutions within the nanofluidic model.
The model may find its use in neurobiological research, development of the
AQP4-targeted drug therapy, optimization of the intrathecal drug delivery to
the brain tumors, in a research on a broad spectrum of
water-metabolic-disorder-related conditions. The nanofluidic mechanism of brain
water metabolism makes it possible to see in a new light the events taking
place in the ISS. It solves a number of issues inherent to the
diffusion-barrier theory that has been unaccounted for so far. A criticism
coming from Verkman’s group concerning fluid flow in the nanodimensional ISS
demonstrates unlikeliness of this event due to high energy demands [43].
Unfortunately, the authors routinely used the no-slip Navier-Stokes approach to
model water flow through the nanodimensional ISS. This basic approach needs to be
reconsidered within the slip-flow paradigm as the nanodimensionality of the ISS
demands. A controversy about the role of AQP4 in water moment across the BBB
presents another problem of the diffusion-barrier theory. Abundantly expressed
in the astrocyte end-feet membranes enveloping the capillaries, the nanochannel
AQP4 controls, according to various experimental data, water exchange across
the BBB and, hence, water movement in the brain [26-34]. But the role of AQP4
as a kinetically limiting step, on one hand, and the diffusion-barrier function
of the ISS, on the other, present two incompatible views. The ISS
diffusion-barrier, as the slowest step of the two, should have assumed a
kinetically limiting role in the overall water moment thus making AQP4 redundant.
The nanofluidic approach solves this controversy asserting the kinetically
limiting role of AQP4.
MORE ON THE
DIFFERENCES BETWEEN BULK AND NANOCONFINED WATER
Dimensionality of compartments changes the
properties of the contained fluids. Following this dictum, the ISF, presenting
the nanoconfined water and the bulk water of the CSF are not identical systems.
Physical properties of nanoconfined liquids strikingly differ from those in
bulk phase. This dramatically affects biophysical and biochemical events taking
place in respective medium. Taking into account those differences becomes
highly relevant to brain physiology and pathology. It is not surprising that
the nanoconfined systems have attracted keen interest in recent years [1-4].
Apart from the enhanced fluid flow phenomenon, there are a number of other
surprising parameters peculiar to the nanoconfined water. Of those, the
dielectric permittivity is probably one of the most important parameters for
the events taking place in the brain interstitial space and for studying and
modeling molecular action mechanisms in nanomedicine. Dielectric properties of
water in nanoconfinement are significantly different from those of the
bulk-state water. The dielectric constant of nanoconfined water captured between
too plane surfaces is anisotropic. Molecular dynamics simulations demonstrate
that it is surprisingly low in the perpendicular direction (ε ┴ ≈10)
and very high in the axial direction (ε║≈700) with the isotropic
dielectric constant for bulk water ≈ 80 [68,69]. The microscopic structure of
water changes depending on the distance from the pore wall and temperature [70].
The nanoconfined water may not be considered a homogeneous fluid but is rather
a heterogeneous system with ε value depending on the direction and
hydrophobicity of the bounding surfaces. Enzyme catalysis, chemical reactions
and other physico-chemical processes taking place in nanoconfined spaces are
receiving increasing attention due to their importance to biology [71,72]. An
important fact is that the thermodynamic activity of nanoconfined water is
different from that of the bulk water [73]. Nanoconfined water affects
profoundly catalytic reactions, the energetic and the reaction mechanisms, the
properties of biomolecules, DNA conformation, protein folding, to list a few,
while its properties play critical roles in a wide range of biological
processes [73-76]. Kinetics of enzymatic reactions in nanoconfinement may
significantly deviate from the Michelis-Menten behavior observed in bulk water
solutions. This deviation is reversible and disappears when confinement is
released by return to the bulk state [73]. The effects of nanoconfinement on
enzyme catalysis depend on the size of the confinement where the reaction
occurs. The effects of spatial confinement, which is especially relevant to
living systems, might be viewed as a new mechanism of metabolism control [77,78].
A research on solubility of gases in nanoconfined fluids has demonstrated that
bulk-water Henry constants are no longer applicable at nanoscale. There is
observed, instead, a striking increase in solubility defined by the term “oversolubility”.
This may result in large uptakes of gases as high as a few hundred times over
expected from bulk solubility [79]. The molecular dynamic simulations and
experimental evidence demonstrated an increase of oxygen solubility in water
under confinement by a factor of 5-10 [80-82]. Solubility increase by factor 15
was found for CO2 [80].
CONCLUSION
Paradigm shift from the diffusion-barrier
concept in the brain water metabolism to the slip-flow nanofluidic approach
asks for awareness of the fluid behavior principles in nanoconfined spaces, the
new fluid properties and their effects on the solvents. Overwhelming share of
biochemical and biophysical knowledge has been obtained so far using bulk
water. Usually it would be diluted solutions where thermodynamic activity
coefficients for respective solutes, as well as for water, would be assigned
unity. Already from rather limited information given in this review one can see
how dramatically different the properties of the nanoconfined water are from
those of the bulk water. All that should be taken into account while
considering brain water metabolism and other events taking place in the brain
NFD. At present an urgent problem is development technologies and
instrumentation to boost further research in the nanoconfined ISS in vivo. The proverbial Genie, called
Nanofluidics, is now out of the bottle. Implementation of new interdisciplinary
knowledge and its translation into basic and clinical research would open wide
perspectives in our understanding of brain physiology, pathology and therapy.
It holds in store a promise of fascinating research along with new challenges.
ACKNOWLEDGEMENT
The author
acknowledges financial support from the National Academy of Sciences of Belarus
through grant 3.09-2016-20 of State Research Programme “Convergency-2020”.
CONFLICT OF INTEREST DECLARATION
The author declares no competing financial interests.
1. Lei Y, Han H, Yuan F, Javeed A,
Zhao Y (2017) The brain interstitial system: Anatomy, modeling, in vivo measurement and applications.
Prog Neurobiol 157: 230-246.
2. Nicholson C, Hrabetova S (2017)
Brain extracellular space: The final frontier of neuroscience. Biophys J 113:
2133-2142.
3. Simon MJ, Iliff JJ (2016)
Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative,
neurovascular and neuroinflammatory disease. Biochim Biophys Acta 1862:
442-451.
4. Bjorefeldt A, Illes S, Zetterberg
H, Hanse E (2018) Neuromodulation via the cerebrospinal fluid: Insights from
recent in vitro studies. Front Neural
Circuits 12: 5.
5. Nicholson C, Kamali-Zare P, Tao L
(2011) Brain extracellular space as a diffusion barrier. Comput Vis Sci 14:
309-325.
6. Nicholson C (2007) Modeling brain
extracellular space from diffusion data. Diffusion Fundamentals 6: 75.1-75.15.
7. Abgrall P, Nguyen NT (2009)
Nanofluidics. Artech House.
8. Mitra SK, Chakraborty S (2011)
Microfluidics and Nanofluidics Handbook. Chemistry, Physics and Life Science
Principles. CRC Press, Taylor & Francis Group.
9. Thorne RG, Nicholson C (2006) In
vivo diffusion analysis with quantum dots and dextrans predicts the width of
brain extracellular space. PNAS 103: 5567-5572.
10. Johanson CE, Duncan JA, Klinge PM,
Brinker T, Stopa EG, et al. (2008) Multiplicity of cerebrospinal fluid
functions: New challenges in health and disease. Cerebrospinal Fluid Res 5: 10.
11. Hua J, Qin Q, Pekar JJ, Van Zijl
PC (2011) Measurement of absolute arterial cerebral blood volume in human brain
without using a contrast agent. NMR Biomed 24: 1313-1325.
12. Palomares JA, Tummala S, Wang DJ,
Park B, Woo MA, et al. (2015) Water exchange across the blood-brain barrier in
obstructive sleep apnea: An MRI diffusion-weighted pseudo-continuous arterial
spin labeling study. J Neuroimaging 25: 900-905.
13. Hemantha TA, Sethaput T (2016)
Quantification of CSF velocity through the narrowest point in aqueduct of
sylvia for normal and normal pressure hydrocephalus patient by CFD analysis.
Int J Pharm Pharm Sci 8: 52.
14. Mawatari K, Tsukahara T, Kitamori
T (2012) Extended-nanofluidic systems for chemistry and biotechnology. Imperial
College Press Distributed by World Scientific.
15. Mawatari K, Kazoe Y, Shimizu H,
Pihosh Y, Kitamori T (2014) Extended-nanofluidics: Fundamental technologies,
unique liquid properties and application in chemical and bio analysis methods
and devices. Anal Chem 86: 4068-4077.
16. Damkier HH, Brown PD, Praetorius J
(2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:
1847-1892.
17. Pollay M (2010) The function and
structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 7:
9.
18. Spector R, Snodgrass SR, Johanson CE (2015) A
balanced view of the cerebrospinal fluid composition and functions: Focus on
adult humans. Exp Neurol 273: 57-68.
19. Sakka L, Coll G, Chazal J (2011)
Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head
Neck Dis 128: 309-316.
20. Brinker T, Stopa E, Morrison J,
Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS
11:10.
21. Iliff JJ, Thrane AS, Nedergaard M
(2017) The glymphatic system and brain interstitial fluid homeostasis. In
Primer on Cerebrovascular Diseases, pp: 17-25.
22. Benveniste H, Liu X, Koundal S,
Sanggaard S, Lee H, et al. (2019) The glymphatic system and waste clearance
with brain aging: A review. Gerontology 65: 106-119.
23. Semyachkina-Glushkovskaya O,
Postnov D, Kurths J (2018) Blood-brain barrier, lymphatic clearance and
recovery: Ariadne's thread in labyrinths of hypotheses. Int J Mol Sci 19: 3818.
24. Carare RO, Bernardes-Silva M,
Newman TA, Page AM, Nicoll JA, et al. (2008) Solutes, but not cells, drain from
the brain parenchyma along basement membranes of capillaries and arteries:
Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol
Appl Neurobiol 34: 131-144.
25. Orešković D, Radoš M, Klarica M
(2017) New concepts of cerebrospinal fluid physiology and development of
hydrocephalus. Pediatr Neurosurg 52: 417-425.
26. Bulat M, Lupret V, Orehković D,
Klarica M (2008) Transventricular and transpial absorption of cerebrospinal
fluid into cerebral microvessels. Coll Antropol 32: 43-50.
27. Oreskovic D, Radoš M, Klarica M
(2017) Role of choroid plexus in cerebrospinal fluid hydrodynamics.
Neuroscience 354: 69-87.
28. Kamali-Zare P, Nicholson C (2013) Brain
extracellular space: Geometry, matrix and physiological importance. Basic Clin
Neurosci 4: 282–286.
29. Sykova E, Nicholson C (2008)
Diffusion in brain extracellular space. Physiol Rev 88: 1277-1340.
30. Hrabetova S, Cognet L, Rusakov DA,
Nägerl UV (2018) Unveiling the extracellular space of the brain: From
super-resolved microstructure to in vivo
function. J Neurosci 38: 9355-9363.
31. Nicholson C (2001) Diffusion and
related transport mechanisms in brain tissue. Rep Progr Phys 64: 815-884.
32. Albargothy NJ, Johnston DA,
MacGregor-Sharp M, Weller RO, Verma A, et al. (2018) Convective
influx/glymphatic system: Tracers injected into the CSF enter and leave the
brain along separate periarterial basement membrane pathways. Acta Neuropathol
136: 139-152.
33. Iliff JJ, Wang M, Liao Y, Plogg
BA, Peng W, et al. (2012) A paravascular pathway facilitates CSF flow through
the brain parenchyma and the clearance of interstitial solutes, including
amyloid beta. Sci Transl Med 4: 147ra111.
34. Morris AW, Sharp MM, Albargothy
NJ, Fernandes R, Hawkes CA, et al. (2016) Vascular basement membranes as
pathways for the passage of fluid into and out of the brain. Acta Neuropathol
131: 725-736.
35. Lam MA, Hemley SJ, Najafi E, Vella
NGF, Bilston LE, et al. (2017) The ultrastructure of spinal cord perivascular
spaces: Implications for the circulation of cerebrospinal fluid. Sci Rep 7:
12924.
36. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A,
Plog BA, et al. (2013) Cerebral arterial pulsation drives paravascular
CSF-interstitial fluid exchange in the murine brain. J Neurosci 33:
18190-18199.
37. Ma Q, Ineichen BV, Detmar M,
Proulx ST (2017) Outflow of cerebrospinal fluid is predominantly through
lymphatic vessels and is reduced in aged mice. Nat Commun 8: 1434.
38. Mase MHE, Yamada H, Oshima N,
Aoyama K, Hibino S, et al. (2016) Water turnover in brain and ventricles in
normal volunteers and patients with idiopathic NPH: dynamic PET study using H2O.
16th International Symposium on Intracranial Pressure and
Neuromonitoring, Cambridge, pp: 119-120.
39. Cserr HF, Cooper DN, Suri PK,
Patlak CS (1981) Efflux of radiolabeled polyethylene glycols and albumin from
rat brain. Am J Physiol Renal Physiol 240: F319-F328.
40. Szentistvanyi I, Patlak CS, Ellis
RA, Cserr HF (1984) Drainage of interstitial fluid from different regions of
rat brain. Am J Physiol Renal Physiol 246: F835-F844.
41. Ichimura T, Fraser PA, Cserr HF
(1991) Distribution of extracellular tracers in perivascular spaces of the rat
brain. Brain Res 545: 103-113.
42. Khaled ARA, Vafai K (2003) The
role of porous media in modeling flow and heat transfer in biological tissues.
Int J Heat Mass Transfer 46: 4989-5003.
43. Jin BJ, Smith AJ, Verkman AS
(2016) Spatial model of convective solute transport in brain extracellular
space does not support a “glymphatic” mechanism. J Gen Physiol 148: 489-501.
44. Holter KE, Kehlet B, Devor A,
Sejnowski TJ, Dale AM et al. (2017) Interstitial solute transport in 3D
reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl
Acad Sci U S A 114: 9894-9899.
45. Kyrtsos CR, Baras JS (2015)
Modeling the role of the glymphatic pathway and cerebral blood vessel
properties in Alzheimer's disease pathogenesis. PLoS One 10: e0139574.
46. Kinney JP, Spacek J, Bartol TM,
Bajaj CL, Harris KM, et al. (2013) Extracellular sheets and tunnels modulate
glutamate diffusion in hippocampal neuropil. J Comp Neurol 521: 448-464.
47. Mestre H, Hablitz LM, Xavier ALR, Feng W, Zou W, et
al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent
brain. Elife 7.
48. Ray L, Iliff JJ, Heys JJ (2019)
Analysis of convective and diffusive transport in the brain interstitium.
Fluids Barriers CNS 16: 6.
49. Iliff JJ, Lee H, Yu M, Feng T,
Logan J, et al. (2013) Brain-wide pathway for waste clearance captured by
contrast-enhanced MRI. J Clin Invest 123: 1299-1309.
50. Iliff JJ, Chen MJ, Plog BA,
Zeppenfeld DM, Soltero M, et al. (2014) Impairment of glymphatic pathway
function promotes tau pathology after traumatic brain injury. J Neurosci 34:
16180-16193.
51. Mestre H, Kress BT, Zou W, Tinglin
Pu, Murlidharan G, et al. (2017) Aquaporin-4 dependent glymphatic solute
transport in rodent brain. BioRxiv preprint.
52. Eijkel JCT, Van den Berg A (2005)
Nanofluidics: What is it and what can we expect from it? Microfluidics
Nanofluidics 1: 249-267.
53. Sparreboom W, Van den Berg A,
Eijkel JCT (2010) Transport in nanofluidic systems: A review of theory and
applications. N J Physics 12: 1-23.
54. Soltani M, Chen P (2013) Numerical
modeling of interstitial fluid flow coupled with blood flow through a remodeled
solid tumor microvascular network. PLoS One 8: 1-18.
55. Smith AJ, Yao X, Dix JA, Jin BJ,
Verkman AS (2017) Test of the ‘glymphatic’ hypothesis demonstrates diffusive
and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife
6.
56. Bocquet L, Charlaix E (2010)
Nanofluidics, from bulk to interfaces. Chem Soc Rev 39: 1073-1095.
57. Richard R, Anthony S, Aziz G
(2016) Pressure-driven molecular dynamics simulations of water transport
through a hydrophilic nanochannel. Mol Phys 114: 2655-2663.
58. Wu K, Chen Z, Li J, Li X, Xu J, et
al. (2017) Wettability effect on nanoconfined water flow. Proc Natl Acad Sci U
S A 114: 3358-3363.
59. Zhang Y, Tunuguntla RH, Choi PO,
Noy A (2017) Real-time dynamics of carbon nanotube porins in supported lipid
membranes visualized by high-speed atomic force microscopy. Philos Trans R Soc
Lond B Biol Sci 372: 1726.
60. Zhu F, Schulten K (2003) Water and
proton conduction through carbon nanotubes as models for biological channels.
Biophys J 85: 236-244.
61. Tunuguntla RH, Henley RY, Yao YC,
Pham TA, Wanunu M, et al. (2017) Enhanced water permeability and tunable ion
selectivity in sub nanometer carbon nanotube porin. Science 357:792-796.
62. Majumder M, Stinchcomb A, Hinds BJ
(2010) Towards mimicking natural protein channels with aligned carbon nanotube
membranes for active drug delivery. Life Sci 86: 563-568.
63. Cantoni M, Imalini E (2017) Water
flow in carbon and silicon carbide nanotubes. Cornell University, pp: 1-7.
64. Geng J, Kim K, Zhang J, Escalada
A, Tunuguntla R, et al. (2014) Stochastic transport through carbon nanotubes in
lipid bilayers and live cell membranes. Nature 514: 612-615.
65. Li D (2008) Encyclopedia of
microfluidics and nanofluidics. Springer.
66. Titovets E (2018) Novel
computational model of the brain water metabolism introducing an
interdisciplinary approach. J Comp Biol Sys 2: 103.
67. Titovets E (2019) Computer
modeling of convective mass transfer of glucose, oxygen and carbon dioxide in
the neurovascular unit. J Comp Biol Sys 4: 101.
68. Zhang C (2018) On the dielectric
constant of nanoconfined water. J Chem Phys 148: 156101.
69. Renou R, Szymczyk A, Maurin G,
Malfreyt P, Ghoufi A (2015) Super permitivity of nanoconfined water. J Chem
Phys 142: 184706.
70. Stanley HE, Buldyrev SV, Kumar P,
Mallamace F, Mazza MG, et al. (2011) Water in nanoconfined and biological
environments. J Non-Crystalline Solids 357: 629-640.
71. Munoz-Santiburcio D, Marx D (2017)
Chemistry in nanoconfined water. Chem Sci 8: 3444-3452.
72. Urban PL (2014) Compartmentalised
chemistry: From studies on the origin of life to engineered biochemical
systems. N J Chem 38: 5135-5141.
73. Jonchhe S, Pandey S, Emura T,
Hidaka K, Hossain MA, et al. (2018) Decreased water activity in nanoconfinement
contributes to the folding of G-quadruplex and i-motif structures. Proc Natl
Acad Sci U S A 115: 9539-9544.
74. Rinaldo P (2017) Effects of
nanoconfinement on catalysis. Springer International Publishing.
75. Vallooran JJ, Assenza S, Mezzenga
R (2019) Spatio-temporal control of enzyme-induced crystallization under
lyotropic liquid crystal nanoconfinement. Angew Chem Int Ed Engl 58: 7289-7293.
76. Wang H (2011) NMR study of water
in nanoscopic confinement and at the interface of biomolecules. University of
North Carolina.
77. Wang C, Sheng ZH, Ouyang J, Xu JJ,
Chen HY, et al. (2012) Nanoconfinement effects: Glucose oxidase reaction
kinetics in nanofluidics. Chem Phys Chem 13: 762-768.
78. Sun W, Vallooran JJ, Mezzenga R
(2015) Enzyme kinetics in liquid crystalline mesophases: Size matters, but also
topology. Langmuir 31: 4558-4565.
79. Ho LN, Schuurman Y, Farrusseng D,
Coasne B (2015) Solubility of Gases in water confined in nanoporous materials:
ZSM-5, MCM-41 and MIL-100. J Phys Chem C 119: 21547-21554.
80. Bratko D, Luzar A (2008)
Attractive surface force in the presence of dissolved gas: A molecular
approach. Langmuir 24: 1247-1253.
81. Luzar A, Bratko D (2005) Gas
solubility in hydrophobic confinement. J Phys Chem B 109: 22545-22552.
82. Lidon P, Marker SC, Wilson JJ,
Williams RM, Zipfel WR, et al. (2018) Enhanced oxygen solubility in metastable
water under tension. Langmuir 34: 12017-12024.
83. Godin AG, Varela JA, Gao Z, Danné
N, Dupuis JP, et al. (2017) Single-nanotube tracking reveals the nanoscale
organization of the extracellular space in the live brain. Nat Nanotechnol 12:
238-243.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Astronomy and Space Research
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Proteomics and Bioinformatics (ISSN:2641-7561)